Extension
Carbon and Climate
About Carbon
• It is the most important element in our living environment. The structure of carbon, with 4 valence electrons in its outer ring, allow it to form complex compounds, and stable organic molecules, such as sugars, starches, proteins, and nucleic acids.
• Much of plant and animal tissue consists of these complex carbon compounds; 45% of plant matter (in the form of cellulose) and 18% of animal matter is carbon.
• It is a significant part of the food that sustains us.
• Carbon provides the energy that fuels our global economy, in the form of coal, oil, and gas.
• As carbon dioxide, it regulates Earth’s temperature.
Where is Earth’s Carbon?
DEEP UNDERGROUND
Most of Earth’s carbon is stored in rocks and sediments underground, as coal, natural gas, and petroleum.
• Coal was formed during the Carboniferous period, between 300 and 350 million years ago. At that time, there were inland seas, the climate was wet, and forests covered the land.
• The plants, as they decayed over time, became pressed down by the earth, further and further until they formed a layer (seam).
• The plant matter appeared to be immune to the digesting ability of the bacteria at that time, and it was the intense pressure and hot temperature of the earth that formed the hard, blackened substance of concentrated carbon (up to 80%) that we call coal.
• Watch the YouTube video at: https://www.youtube.com/watch?v=BQ_Ethb6_Wk
• Natural gas is found in rock formations, deep underground and often associated with coal.
• Crude oil is formed from the remains of dead organisms (diatoms) such as algae and zooplankton that existed millions of years ago in a marine environment, and were the dominant forms of life on earth at the time.
• All three forms of underground carbon are rich sources of energy. Because they are dug up out of the earth from very ancient deposits, they are referred to as fossil fuels.
EARTH’S SURFACE
Atmosphere
• Carbon is present in the atmosphere as the gas, carbon dioxide. Its concentration, today, is roughly 0.04% of all the gases.
• Other gases include nitrogen (N2 ) (78%), oxygen (O2 ) (21%), and argon (Ar) (0.93%), and tiny amounts of other gases, such as methane (CH4 ), helium (He), neon (Ne), ozone (O3 ), nitrous oxide (N2 O) and water vapour.
Oceans
• Oceans are a vast source of carbon, holding 150 times more than the carbon dioxide in the atmosphere.
• Aerating ocean water introduces the following chemical reaction between water and carbon dioxide,
• CO2 + H2O —> H2 CO3 (carbonic acid).
• Carbonic acid lowers the pH of the water by ionising into HCO3- + H+ (bicarbonate and acid ions).
• This process has maintained seawater at a constant level of acidity, with a pH of 8.2 (today at 8.1).
• On the ocean’s bed, carbon is in the form of calcium carbonate (CaCO3 ), the remains of shells and skeletal remains of sea creatures.
• These shells and other sunken organic materials ultimately form the sediment and rocks lining the ocean floor.
Soil
A significant amount of carbon is sequestered in the soil. It is in the form of living matter, such as soil invertebrates, microfauna and fungi, and as complex and simple organic molecules of decayed matter.
Movement of Carbon
For information on the movement of carbon throughout the ocean, atmosphere, and biosphere, see the website: https://earthobservatory.nasa.gov/features/ CarbonCycle
Carbon is not stationary. It moves very slowly, over geological time, throughout Earth’s nonliving carbon reservoirs, either as a gas (carbon dioxide, in soluble ions, such as bicarbonate (HCO3-), an inorganic compound, or a complex organic molecule.
Carbon compounds are continually cycling between living plants and animals, in the processes of photosynthesis and respiration (see Year 7). Dead animal and plant matter, with the digestive ability of micro-organisms, returns this carbon to the surface soil layers.
GEOLOGICAL (SLOW) CARBON CYCLE
Carbon, as atmospheric carbon dioxide, combines with water to form carbonic acid.
• When oceans evaporate, this compound falls to the surface in rain.
• The acid rain dissolves rocks, a process called chemical weathering, releasing ions from carbon, which are carried in rivers back to the oceans.
Carbon stored in rock, is released during tectonic movement.
• Earth’s land and ocean surfaces sit on several moving crustal plates. When the plates collide, one sinks beneath the other, and the rock it carries melts under the extreme heat and pressure.
• The heated rock recombines into silicate minerals, releasing carbon dioxide, which is vented to the atmosphere through volcanos. The larval flow covers the land with fresh silicate rock to begin the cycle again.
• It takes a few hundred thousand years to rebalance the slow carbon cycle through chemical weathering.
It is calculated that about 10 to 100 million metric tons of carbon moves between the rocks, soil, ocean and atmosphere each year.
Teacher Note: An interesting statistic – at present, volcanoes emit between 130 and 380 million metric tons of carbon dioxide per year. For comparison, humans emit about 30 billion tons of carbon dioxide per year by burning fossil fuels.
BIOLOGICAL (QUICK) CARBON CYCLE
The biological carbon cycle describes the relatively quick flow of carbon between the atmosphere and Earth’s ecosystems.
• Plants take in carbon, as atmospheric carbon dioxide, and convert it into complex sugars during the process of photosynthesis.
• Carbon circulates throughout the animal and plant food chains, with carbon dioxide as the end-product of respiration, and is returned to the atmosphere.
• When plants and animals die, their bodies decay as a result of soil bacteria and fungi, which break down complex molecules, returning carbon to the soil to be recycled as food for bacteria and fungi, and plant nutrients.
Carbon Cycle. Photo: University Corporation for Atmospheric Research.
Ask Questions About Carbon
• Is it a molecule, or an atom?
• Where would one look to find it? (In plants, animals, atmosphere, under the ground, in the ocean)
• What are the stores of carbon underground? (coal, oil, and natural gas in rocks; and as peat)
• How do stores reach the Earth’s surface? (extraction; volcanos)
• How much carbon is in the ocean? (150 times that in the atmosphere)
• In what form is it in the ocean? (carbonic acid, calcium carbonates, skeletal remains of animals)
• What do plants do with carbon? (incorporate it into their tissues as cellulose; synthesise sugars)
• From where do plants get carbon? (atmosphere, as the gas carbon dioxide)
• What do animals do with carbon? (eat it as sugars, incorporate it into body tissues; breathe it out into the atmosphere as carbon dioxide).
• Do plants put carbon into the atmosphere? (yes, when they respire, similar to animals).
CARBON DIOXIDE AND CLIMATE CHANGE
Watch YouTube clip with Al Gore - An Inconvenient Truth. https://www.youtube.com/results?search_ query=al+gore+documentary+an+ inconvenient+truth+youtube (TOOLBOX)
For a one page, clear explanation of the causes of climate change: https://climate.nasa.gov/causes/
What Keeps Our Planet Warm?
Carbon dioxide and methane are the principal gases that interact with the radiant heat energy from the Sun, trapping the heat and keeping our planet warm enough for life.
Both molecules have the ability to absorb infra-red energy, in the form of photons, from the Sun’s radiation energy.
• As the molecule, CO2 soaks up the heat energy, it vibrates and re-emits the energy back in all directions. About half the energy goes out into space, and about half of it returns to Earth as heat, trapped under the atmospheric layers, which contributes to a ‘greenhouse effect.’
• Methane (CH4 ) is the natural gas that we use as heating. It absorbs infra-red energy, about 120 times more effectively than CO2 . Although in very small concentration in the atmosphere, a significant amount of methane exists under the permafrost in Arctic regions, as methane hydrate. This molecule is an ice-like substance formed when CH4 and water combine at low temperature (-25Co) and under moderate pressure.
The natural ‘greenhouse effect’ is very important; without it the average temperature on the surface of the planet would be about -18°C and human life would not exist.
Greenhouse effect in a Bottle (explained). Scroll down to How Exactly Does Carbon-dioxide Cause Global Warming.
https://news.climate.columbia.edu/2021/02/25/ carbon-dioxide-cause-global-warming/
What Increases the Levels of Greenhouse Gases?
• Carbon dioxide levels in the atmosphere increase when fossil fuels are burned to power up factories, power-plants, and vehicles • Methane is produced from food waste in landfill, gas production in cattle and termites.
• With increasing temperatures, Arctic ice melts, releasing the methane stored in the permafrost into the atmosphere.
• Nitrous oxide (N2 O) also warms the atmosphere. Its potency and relatively long life make N2 O a dangerous contributor to climate change.
• Levels of N2 O increase after biomass burning. Significant quantities are produced through large scale burning of woodlands, and agricultural waste.
Are Levels of Carbon Dioxide Rising?
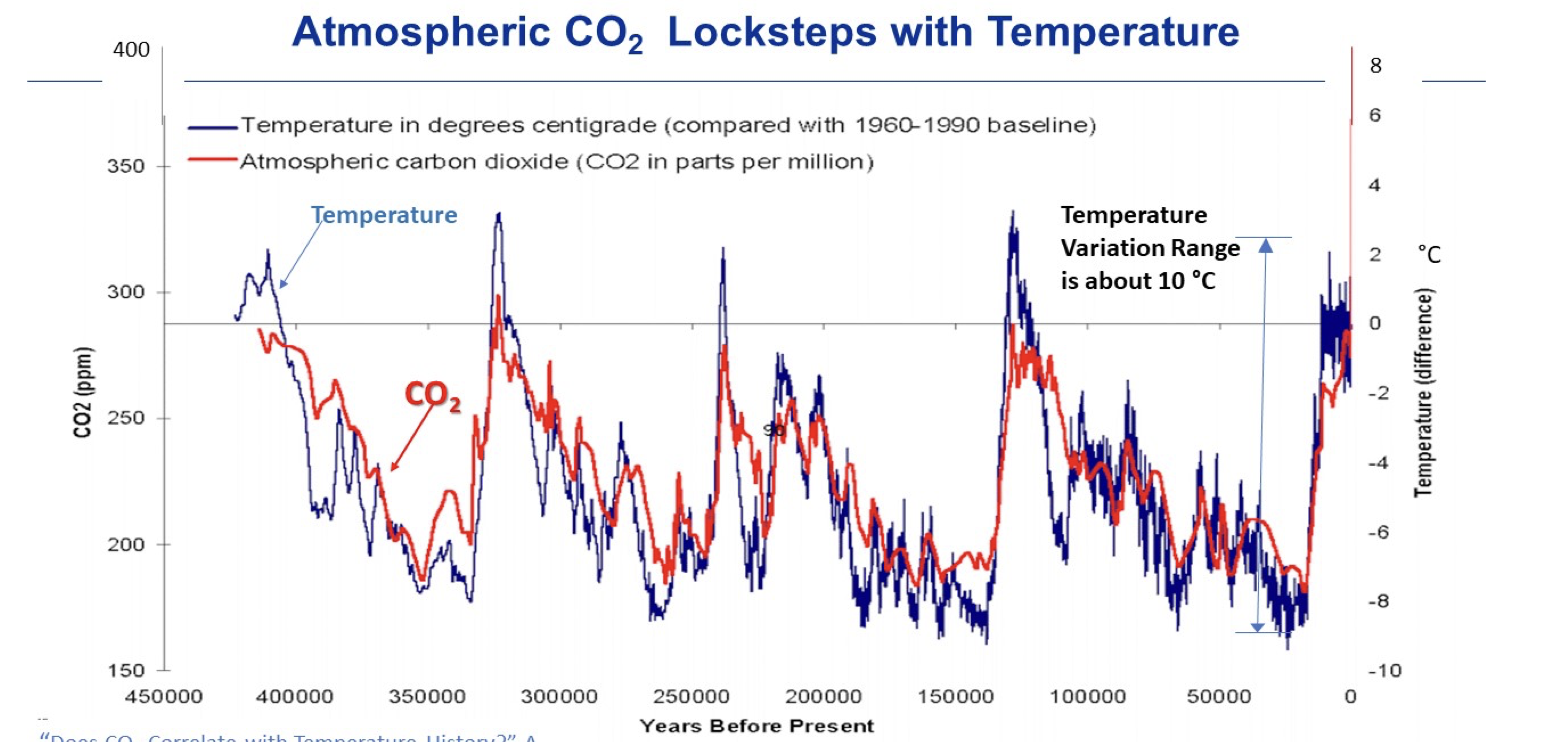
• Scientists have measured the concentration of carbon dioxide in ice cores from 800,000 years ago at the Mauna Loa Volcanic Observatory, and up to the present, with NASA’s Orbiting Carbon Observatory (OCO). Carbon dioxide has fluctuated regularly every 100,000 years, between 180 ppm during ice-ages (glacial periods) to 275 ppm during warm periods (interglacials).
• Around 1770, the level of atmospheric CO2 was 0.0275% (or 275 ppm), similar to the previous warm periods, and atmospheric levels of CO2 began to rise. This was during the Industrial Revolution, when industries were fuelled by the burning of coal and other fossil fuels, rich in carbon. When carbon in coal is burnt, (or oxidised), oxygen combines with carbon to form carbon dioxide.
• Since then, the levels have risen sharply. About 70 years ago, the level had risen to 315 ppm; ten years ago to 387 ppm; and in the last 10 years, today, in 2023, the concentration of CO2 has reached its highest ever at 421 ppm. This rise is out of proportion to all previous rises in CO2 and scientists have correlated this unprecedented level of CO2 with the burning of fossil fuels during the last 250 years.
Is Earth’s Temperature Rising?
• Modern scientists and engineers have explored Earth’s temperature by drilling into the ice sheets that cover Antarctica and Greenland. Thousands of years of snow have compressed into thick slabs of ice and the resulting ice cores can be more than 3 km long, extending back in time of a staggering 800,000 years.
• Scientists use the chemistry of the water molecules in the ice layers to see how the temperature has varied through the millennia.
• The average global temperature on Earth has increased by at least 1.1° Celsius (1.9° Fahrenheit) since 1880. The majority of the warming has occurred since 1975, at a rate of roughly 0.15 to 0.20°C per decade.
• This doesn’t sound like much, but a one degree global change is significant because it takes a vast amount of heat to warm all of the oceans, the atmosphere, and the land masses by that much. In the past, a one-to two-degree drop was all it took to plunge the Earth into an Ice Age.
Are Rising Levels of Carbon Dioxide Associated With Rises in Temperature?
• We have known about the greenhouse effect for well over a century. About 150 years ago a physicist called John Tyndall used laboratory experiments to demonstrate the greenhouse properties of CO2 gas. Some years later, the Swedish chemist Svante Arrhenius first calculated the greenhouse effect of carbon dioxide in our atmosphere and linked it to past ice ages on our planet.
• The ice layers in the ice cores also trap tiny bubbles from the ancient atmosphere, allowing scientists to measure prehistoric carbon dioxide levels directly and correlate with the fluctuations in global temperature over time.
See: https://www.epa.gov/climate-change for background information on Climate Change. See: https://archive.epa.gov/climatechange/kids/ documents/temp-and-co2.pdf for student
Atmospheric CO2 levels in the last 800,000 years In: https://climate.nasa.gov/vital-signs/carbon-dioxide/ (TOOLBOX)
World’s temperature since 1880. From: https://earthobservatory.nasa.gov world-of-change/global-temperatures (TOOLBOX)
Correlation of temperature with CO2 levels in the last 400,000 years (TOOLBOX). Figure from A. Watts and M. Pacnik, in Valone 2021.
Activity
Construct a Circular Diagram Featuring the Movement of Carbon Throughout Earth’s Systems:
Include where carbon is found, what form it is in, its concentration (where known), and indicate the organic processes such as photosynthesis, respiration, and plant decomposition.
Activity
From the website above, research the data and construct graphs showing, (i) the amount of carbon dioxide in Earth’s atmosphere over long time, and (ii) Earth’s surface temperature over time:
From your 2 graphs, note any correlation between a rise in temperature with a rise in CO2 concentration. Formulate your conclusion and record.
• This activity also serves as a revision exercise in constructing graphs. Students revise the names of the horizontal x-axis (the abscissa, as the independent variable) and the vertical y-axis (ordinate or dependent variable).
• For clarification, the known time intervals ‘fix’ the x-axis – it is independent. Measuring how CO2 levels ‘vary’ during the fixed time levels, means they are dependent on the time when they were measured – hence, they are the dependent variable.
From: https://mathbench.umd.edu/modules/ visualization_graph/page02.htm
Activity
Create a Table of Knowledge About Climate Change:
Research on the Internet, the answers to the following questions about Climate Change:
• Where is carbon dioxide stored?
• Where is methane stored?
• Why are both called ‘greenhouse gases’?
• What are the sources of carbon dioxide and methane in the atmosphere?
• What will happen when the arctic permafrost melts?
• What methods are used to lower the carbon dioxide concentration in the atmosphere?
Activity
What can Humans do to Reduce the Amount of Carbon Dioxide in the Atmosphere?
1. Students can help reduce their contribution to the greenhouse gas effect by ensuring that they always compost their food waste to reduce the amount going to landfill. This is a simple action with a profoundly positive effect on the environment.
2. Walking and bike riding reduce our use of fossil fuel combustion. Walk or Ride to school days could be held more frequently
3. Burning off in the backyard and frequently burning the bush increase the load of these gases in the atmosphere. Ban the incinerator and report bushfires.
4. Organise Caring for Country get-togethers with the local community to listen to ideas and form plans for improving the health of our natural environment.
Glossary
Infra-red energy – energy in the Sun’s radiation spectrum between 620 – 750nm
Photons – energy-containing particles from the Sun
Greenhouse effect – The trapping of heat in the inner layer of Earth’s atmospheres, similar to the action of a garden greenhouse.
Methane hydrate – a crystalline solid that consists of a methane molecule surrounded by a cage of interlocking water molecules.
Correlation –a statistical relationship, whether causal or not, between 2 random variables (or bivariate data, as in the exercise above).
Reference
1. Valone, T.F., 2021, Linear global temperature correlation to carbon dioxide level, sea level and innovative solutions to a projected 6oC warming by 2100. Journal of Geoscience and Environmental Protection. 9.