Module
The Need for Healthy Soils
The Need For Healthy Soil
Healthy soils are vital for life. They are central to our agricultural industry, our human health, our food, and water security. The immense diversity of microorganisms and animals that live belowground contributes significantly to this health, shaping above ground biodiversity and the functioning of terrestrial ecosystems. Scientists are now discovering the importance of belowground ecosystems in supporting the resilience of natural ecosystems to recover from disturbance. In combination with aboveground factors, healthy soil helps to buttress the landscape from natural disasters, such as those that ensue from climate change [3, 4].
The economic services provided by Australia’s soil has an estimated value equivalent to about $930 billion per year, with agricultural production contributing approximately $63 billion per year to the Australian economy.
Show the following video as an instructive introduction to soil.
The Special Case of Wheatbelt Soils
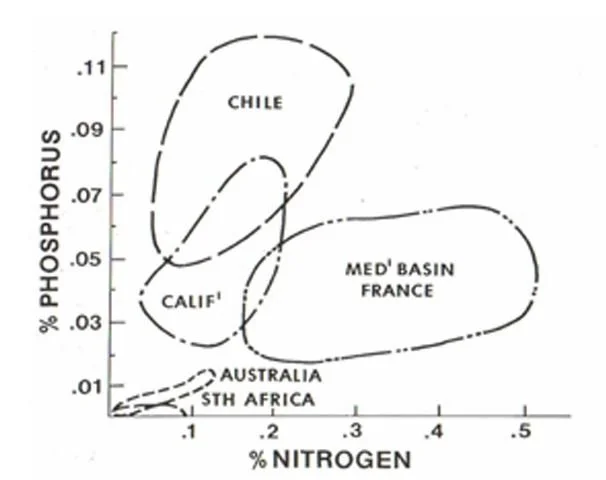
Compared with soils in other regions, (see Figure), Wheatbelt soils share with those of South Africa, as being the most impoverished. Leaching and weathering over long time has drained soil of the elements that are essential for plant growth, such as nitrogen (N), phosphorus (P), and potassium (K), known as NPK.
Other important nutrients are calcium, (Ca), magnesium (Mg), and sulphur (S). Plants also need small quantities of iron, manganese, zinc, copper, boron, selenium, and molybdenum, known as trace elements, because only traces are needed by the plant.
Comparison of nitrogen and phosphorus content in world soils, Don Bradshaw
Oral McGuire (Ballardong Elder) says...
“In the 1960s, in Ballardong country, farming was pretty good. We got regular and reliable rain. My dad drove tractors, and I sat on the tractor with him watching the beautiful soils being tilled and seeded. The land produced crops that were taller than my dad - he was 6 foot two. Today, I drive past those same paddocks and the crops are lucky to rise higher than the fence line, which is about 3 foot.
Two hundred years ago, the land in [Ballardong country] was very well vegetated. Today, we have horrible, compressed, compacted soil. We’ve got massive problems with erosion. There’s a [significant] cost [associated with] healing that sort of country.”
From: ‘Oral McGuire: Cultivating Connections’. An interview with Rosie Halsmith [5]
Who Enriches the Soil
The Extremely Little Guys
Bacteria and fungi are the most important organisms in soil, in terms of (i) their composition of organic matter and storage of carbon, (ii) the interactions they have with plants and other micro-organisms and (iii) their decomposition of plant and animal material to its constituent compounds is the base of the soil food web.
Bacteria
Bacteria are the most abundant microbes in the soil. They are single celled organisms, and there can be billions of bacteria in a single gram of soil. There are many types that have different roles.
• Decomposers play an important role in the early stages of decomposition of organic materials
• Nitrogen fixers, such as Rhizobium bacteria that live in special root nodules on legumes, extract nitrogen gas from the air and convert it into forms that plants can use. Nitrogen fixers add the equivalent of more than 100 kg/ha per year of nitrogen to the soil.
• Disease suppressors: release antibiotic substances to suppress particular competitors.
• Actinobacteria help to slowly break down humates and humic acids in soils, and prefer non-acidic soils with pH higher than 5.
• Sulphur oxidisers, such as Thiobacillus bacteria, can covert sulphides (common in soil minerals but largely unavailable to plants) into sulphates, a form plants can use.
Fungi
• Decomposers: are essential for breaking down woody organic matter.1 They play an important role in immobilising and retaining nutrients in the soil. The organic acids they produce help create soil organic matter that is resistant to degradation.
• Mutualists (symbiotic relationships with plant roots): Arbuscular mycorrhiza (VAM) described below.
• Pathogens: such as Phytophthora, penetrate the plant and decompose the living tissue, leading to weakened or dead plants. Soils with high biodiversity can suppress soil-borne fungal diseases.
From: https://www. soilquality.org. au/factsheets/soil-bacteria-and-fungi-nsw
Soil microfauna. Photo, M. Ilardi
The Little Guys (0.2-200mm)
Consumers and Fragmenters
• Mites, with 8 legs, belong to the Class Arachnida, and Order, Acarina. There are many different species of mite; their fossil record dates back 400 million years. They are both free-living and parasitic, feeding on almost everything organic in soil, including both plant and animal residue, as well as fungi and bacteria. As important decomposers, mites perform a vital role in soil decomposition, helping to break down organic matter and recycle it as nutrients for plants. In addition, they are a food source for other soil organisms, such as springtails, nematodes, and predatory mites; they are a key species in the soil food web.
• Springtails, belonging to the Class Insecta (having 6 legs), and Order Collembola, are also an ancient group. They are common in all surface soils and litter, about 100,000 per square metre in moist conditions. Although tiny, they are easily recognised by an appendage projecting ventrally from their abdomen. It’s called a furcula. At rest, the springtail keeps it tight, and under tension, against its ventral surface. When released, the furcula snaps against the ground under the animal, flinging it into the air, possibly an escape mechanism? Their main benefit to soils appears to be the breaking up (or fragmentation) of organic matter in the soil and controlling microbial communities.
Tunnellers
• Earthworms eat their way through the soil, creating vast tunnel systems. The worm’s first segment contains its mouth, and as they burrow, they suck in the soil in front of them. Extracting the nutrients from decomposing organic matter, like leaves and roots as they pass through its body, the worm excretes the digested matter as faeces, and together with mucus, this lines the tunnel walls, some of which can be up to 2 metres in depth.
• There are some staggering statistics on the number of earthworms in the world’s soils. These range from 40,000 to 80,000 per hectare, and may be as high as 400,000/ha creating up to 400 km of tunnels, each week!
Charles Darwin was perhaps the first to bring to the world’s attention the importance of worms in the soil, as the subject of his final book – The Formation of Vegetable Mould Through the Actions of Worms (1881).
“…Their passage through the earth aerates the soil and the natural chemistry of their guts renders soil and plant matter into fertile pellets...” Darwin concluded,
“…It may be doubted if there are any other animals which have played such an important part in the history of the world as these lowly organized creatures.”
• Ants are also important soil engineers. By creating their tunnel systems underground, they aerate the soil and allow water penetration.
• Ants forage in the litter, carrying seeds, plant parts, and dead and decayed materials from the topsoil into their nests, thereby enriching soil organic carbon and nitrogen.
• Ants also forage from above-soil plants, such as Acacia sp., harvesting sugar compounds from their extra-floral nectar secretions, the plant’s stems, which are then taken underground[6].
Springtail. Photo, M. Ilard
Activity
Extracting Micro and Macroinvertebrates in Soil:
As much of the microfauna inhabit the litter layer, the standard method of extraction of leaf litter animals involves a heat source. This is usually a light globe above a leaf litter sample sitting on a gauze with some sort of cover to stop the litter drying out rapidly, such as a Berlese-Tullgren apparatus.
Teachers will find a demonstration of how to construct a Berlese-Tullgren apparatus in: https://www.uwa.au/ study/for-teachers/science-resources-for-teachers
Scroll down to Years 1-6, Introduction to Soil Science, Exploring Soil, Extracting Soil Fauna.
Show the video, Extracting Soil Fauna, (PPTS/Videos)
MATERIALS
• Funnel and beaker (made from a 1.5L plastic drink bottle)
• 70ml lidded collecting container
• Mesh (aluminium flywire)
• Gauze swabs
• 40W microscope lamp
• Plaster of Paris and charcoal, dampened (optional) (see TOOLBOX)
• Soil samples A and B
METHOD
1. Prepare the extraction apparatus:
i. Place the collecting container, with its lid off, inside the plastic beaker and rest the funnel over the top.
ii. If using Plaster of Paris and charcoal, place a layer in the base of the collecting container – the dampness (do not soak!) helps to keep the animals alive
iii. Place one layer of mesh and two layers of gauze swabs inside the funnel. These prevent chunks of soil falling into your collecting jar, but still allow the soil animals through as they move away from the lamp’s heat
2. Collect a sample of soil plus litter measuring about 5-8cm across, and to a depth of 5cm with a trowel. Place immediately in a zip-lock plastic bag, or alternative and fasten securely.
3. Empty your soil sample into the funnel, shaking any loose bits of soil from the zip-lock bag.
4. Place the lamp about 7 centimetres above the funnel.
5. Check the equipment daily. As the sample dries out, you should start to see tiny soil animals in the collecting container underneath.
6. If using Plaster of Paris, make sure the base stays slightly damp, otherwise your soil animals will die.
7. Examine under Low Power to identify (see GRAPHICS With my naked eye I saw.., Under the microscope I saw.., Soil fauna. (TOOLBOX)
Tullgren Funnel
The Big Guys
Bioturbators
These vertebrates play an important part in the health of soils [7]. Animals that search for food (forage) in soil, use their long claws and strong forefeet to turn over plant litter and dig shallow scratchings and holes when foraging, a process called ‘turbation’. In digging, they mix organic matter into the soil and spread mycorrhizal fungi and seeds. This top soil disturbance aerates the soil, and allows water to penetrate.
Three significant bioturbators were once widespread in the Wheatbelt; two marsupial mammals, the Brush tailed bettong (Bettongia penicillata) (woylie) and the Southern Brown bandicoot (Isoodon fusciventer) (quenda), and the more prevalent prototherian mammal, the Echidna (Tachyglossus aculeatus) (nyingarn).
• Woylie (bettong) digs for underground insects, worms, and roots, but its main diet is fungi (truffles). It is now rarely found in the Wheatbelt, having been recorded in only two regions, the Upper Warren District, and the Dryandra Woodland. Its numbers have fallen dramatically in recent years and the woylie is now classified as Critically Endangered.
• Quenda, kwenda (Southern Brown bandicoot) is more widespread, although it has lost much of its habitat of dense woodland and around swampy areas. Quendas forage on earthworms, beetles, fungi as well as plant material such as tubers and bulbs. As bioturbators, their effects on soil has been much researched by scientists [8] . When foraging, the quenda leaves behind its foraging pit a mound of soil, called a ‘spoil heap’. When the nutrients were analysed in the spoil heap, such as phosphorus, potassium, sulphur, organic carbon, and microbial activity, they all showed higher levels than in the foraging pit itself. When scientists grew Tuart seedlings (Eucalyptus gomoncephala) in the 2 types of soil (pit and heap), the plants grown in the spoil heap were taller, heavier and grew at a faster rate, earning quenda the classification as ‘ecosystem engineer.’[9].
• Nyingarn (echidna) is widespread across Australia, and can be found under vegetation, sheltering in hollow logs or deserted warrens; or just burrowed under the soil surface. It feeds almost exclusively on ants and termites, using its adapted mouth parts (see Years 5-6 Specialisations). By using accelerometers (devices attached to the animal that measure the distance and speed of travel), scientists measured the distance an echidna travelled each day as 3.6 kilometres in Spring, and 2.7 km in Summer [10].
• The echidna has a different method of bioturbation. It buries itself beneath the soil surface, shifting a significant amount of soil and leaf litter in the process. Scientists calculated the amount of soil a single echidna could move in a day [10], providing some interesting statistics, as follows:
• The animal spends 12% of its day in digging, and with each dig lasting 30 seconds, the animal displaced half its body volume as soil and litter within a minute.
1. The scientists calculated the volume of an echidna, (based on a mean density of 1 g.cm -3) as 3230 cm3 for a 3.23 kg echidna.
2. Therefore, digging for 12% of the day, an echidna could move up to 0.558 m3 of soil and litter (about a wheelbarrow load) in a day.
3. Extrapolating to an annual rate of digging, means an echidna could move 204 m3 in a year (about a square of earth measuring 6 metres long, 6 metres wide, to a depth of 6 metres!).
4. Analysing soil in the foraging pits of echidnas in Eastern Australia, and comparing it with adjacent undisturbed soil, scientists found the pit soil absorbed twice the amount of water and had twice the amount of organic debris and seeds [11]
Echidnas are therefore extremely important contributors to the health of the Wheatbelt (and Australia’s) soil ecosystems.
Watch the YouTube clip showing ‘the love train’ of echidnas, at https://www.science.org.au/curious/video/echidna-love-train
Brush-tailed bettong, Woylie. Photo Brad Leue, AWC
Southern Brown bandicoot, quenda.
Short-beaked echidna, Tachyglossus aculeatus. Photo, Gunjan Pandey
Diagrammatic representation of a ‘spoil’ heap. From Valentine et al, [9]
Activity
Weighing Soil in the Echidna Diggings:
The echidna is a highly adapted mammal (see Yr 5-6, Specialisations). Its feeding apparatus includes an elongated ‘beak’ with which it prods the earth to sense (smell) its food of ants. In doing so, it dislodges the soil.
This activity may require assistance from Aboriginal educators to locate an echidna (nyingarn).
AIM
To measure the digging and turning over of soil by an echidna in measured time.
MATERIALS
• Cotton spools (available from the author)
• 1cm wide tape
• Garden trowels
• Self-seal plastic bags
• Balance
METHOD
1. With assistance from an Aboriginal educator, locate an echidna.
2. Using a length of tape, and a cotton spool, wrap the spool to a spine at the rear of the echidna’s back. The spool unwinds from the interior, so make sure the internal hole of the spool is not covered by the tape.
3. Attach the end of the cotton coming from the inside of the spool to a nearby tree (or stout bush). The procedure is best done early in the morning, or late afternoon, depending on when the echidna is caught.
4. Leave the echidna in its habitat undisturbed.
5. Return one week later (or several days, if practicable) to the site where you tied the end of the cotton thread and follow it to retrace the echidna’s movements.
6. Note particularly where the echidna has stopped to dig. Count the number of holes and collect an equivalent volume of soil in plastic bags.
7. It is likely that the echidna has unwound all of the cotton in the spool over the time (they contain about 300 metres of thread).
8. If not, and it is still attached to the echidna, (which could be in a burrow), remove the spool carefully from the animal, and cut off the amount that has been unwound.
9. The weight of the unwound thread will indicate the distance travelled by the echidna during the number of days, using the following information- 150m of thread weighs 2.718g.
10. Make sure that the echidna is unharmed and makes its way off when released.
CALCULATIONS
1. Weigh all the soil samples and calculate the mean weight. This indicates the weight of soil turned over in the time between fixing the spool and recovery of the spool.
2. From this, knowing the number of holes dug over the time, calculate how much soil one echidna ‘bioturbates’ in a year.
[A recent scientific paper where they radio-tracked echidnas concluded that each echidna moved approximately 200 m3 of soil per year which equates to 600 kg or 0.6 tonnes per year!] [10].
Activity
Write up the Contribution to Soil Health Made by the Animal Kingdom:
From the information above and researching the Internet, outline the role of micro-organisms (including fungi), invertebrates and vertebrates to the maintenance of the ecosystem in Wheatbelt soils. Diagrammatically, this may include a pyramid, with micro-organisms and chemical decomposition at the base, and bioturbators at the apex.