Module 1
Photosynthesis
The Sun as Energy
Show PowerPoint – The Sun, and its energy, Part 1 (TOOLBOX)
MAKING ENERGY FROM HYDROGEN
NUCLEAR FUSION
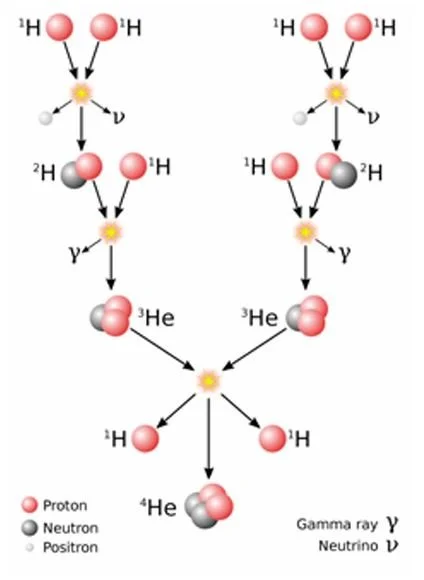
The Sun is a giant nuclear reactor, using hydrogen gas as the fuel. The pressure inside the Sun is so great that 4 atoms of hydrogen (1H) are fused to form 1 atom of helium (4He).
Briefly, a hydrogen atom is composed of a nucleus with a single proton (a positively charged particle) with a negatively charged electron moving around it. A helium atom has a nucleus of 2 protons and 2 neutrons (i.e. 4 particles) and 2 electrons orbiting around the nucleus. In essence, four of the hydrogen atoms are transformed into the 4 particles comprising the nucleus of the helium atom.
Every second, the Sun fuses 620 million metric tons of hydrogen into 616 million metric tons of helium. In the process, 4 million metric tons (4 x 106 metric tons) is generated in the fusion process.
This mass, formed in the core of the Sun, is the source of the energy.
Composed of alpha particles and electromagnetic energy, the amount of energy created is enormous, and is expressed in Albert Einstein’s famous formula, E = mc2, where E represents energy, m is the mass (in this instance, 4 x 106 metric tons), and c is the speed of light (300 x 106m.sec) raised to the power of 2 (or squared).
HOW ENERGY TRAVELS
The energy eventually finds its way to the outside of the Sun and radiates as photons, which may be viewed as either particles or waves. Photons, as light energy, have no mass and travel at the speed of light (299,792,458 metres per second) – close enough to 300,000,000m/s. In wave formation, the frequency of photons (number per unit time) is usually expressed as the distance travelled between successive peaks, in nanometres (1m divided by one thousand million, or 1 x 10-9m). Thus, violet light, where peaks are 350 to 400nm apart, has a greater energy level than red light, where peaks are 600 to 700 nm apart.
Represented as a continuum, the electromagnetic spectrum describes all the kinds of energy in the universe. The lower the frequency, the lower the wave energy; conversely, the greater the frequency of the wave energy, the greater the energy being radiated from the Sun.
A comprehensive reference at:
SEEING ENERGY
Our eyes, with special cells in the retinal layer, are able to detect (and interpret as colour) only wavelengths between 350 to 700 nanometres.
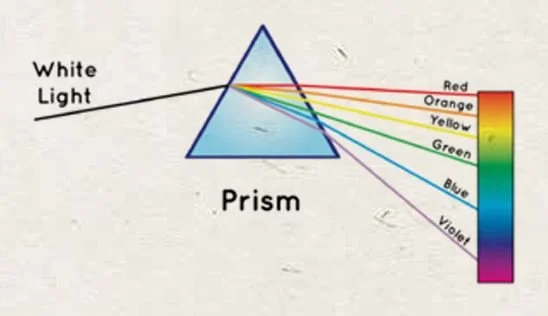
And then, only when the light waves become refracted, as in a rainbow. This is because energy wavelengths can be separated because of their different frequencies. When white light passes through a prism, or a water droplet in a rainbow, the wavelengths become separated as they pass through the different angles created by the prism. This is called refraction.
Other wavelengths are invisible to humans, and can only be measured using instruments (such as thermometers, X-ray machines, and gamma detectors).
LIGHT INTO CHEMICAL ENERGY
Chloroplast
Chlorophyll
Inside each chloroplast are chlorophyll molecules stacked into green coin-like objects, called thylakoids. These structures evolved from early life forms when they were a system of tubular lamellae resembling sacks. Over time, the lamellae have folded into the coin-like structures, and named thylakoids, from the Greek word thylakos, meaning sack.
https://collegedunia.com/exams/chloroplasts-biology-articleid-1698
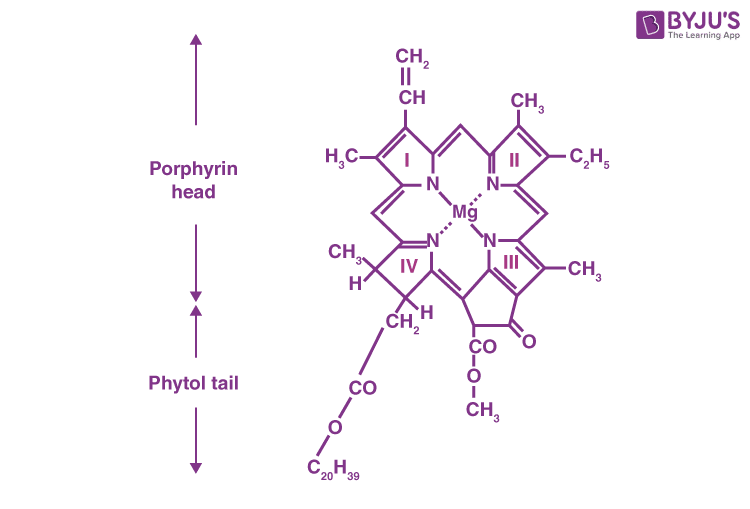
The chlorophyll molecules (see Figure) are arranged such that the long carbon: hydrogen chain (C20 H39 ) is on the outer wall of thylakoids, and acts as a chromophore, absorbing visible light photons. Like the cones and rods in the retina of our eyes, chlorophyll interprets the energy in the electromagnetic spectrum. (see Figure, Absorption spectra of pigments).
However, not all energy in visible light is absorbed into the chlorophyll molecule. Energy wavelengths of 400 to 450 nm and 600 to 700nm are absorbed, but not the energy in wavelengths between 450 and 550nm. These energies are reflected back into the atmosphere by the leaf, giving it (to our eyes) the colour green.
MAGNESIUM AND AN EXCITON
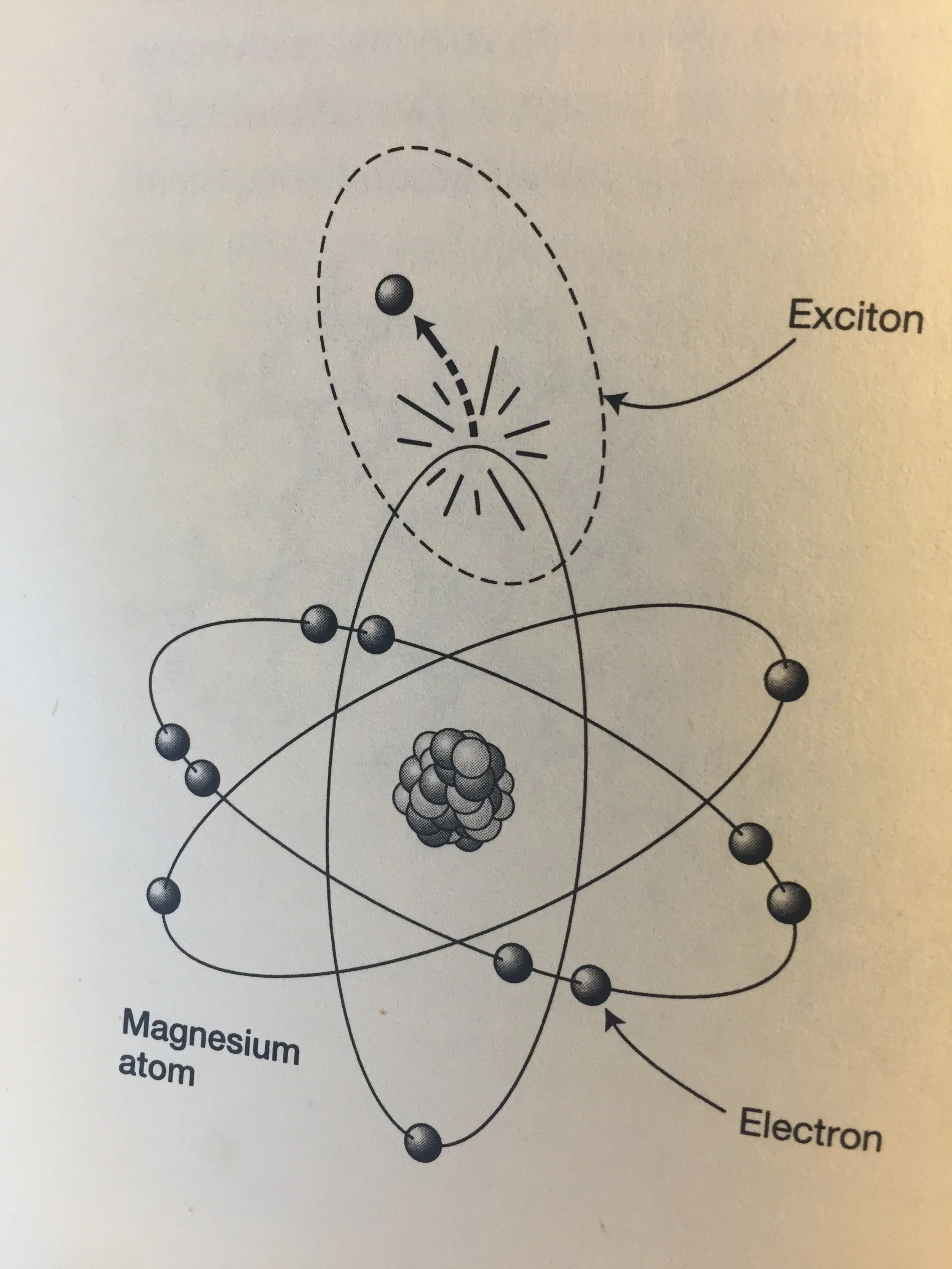
Bound inside each chlorophyll molecule is an atom of magnesium. A magnesium atom consists of a nucleus of 12 positively charged particles (protons), 12 particles with no charge (neutrons) and ‘shells’ of 12 negatively charged orbiting electrons.
An outermost electron is only loosely bound to the rest of the atom. A photon of solar energy is absorbed into the magnesium molecule, exciting the outermost electron, and knocking it into its surrounding carbon cage. This leaves a gap, which can be thought of as a positively charged hole. The concept described here is the creation of a system consisting of the escaped negative electron and the positive hole it has left behind. This binary system is called an exciton. It could be thought of as a tiny battery with positive and negative poles capable of storing energy for later use (1).
Excitons are unstable. One is very quickly stripped from its atom and transferred to neighbouring chemicals, such as the complex protein molecules PSI and PSII. Electron flow between these molecules powers up the molecules ATP and NADPH (see GLOSSARY).
For more about excitons, see:
Activity
Inside a Plant Cell:
A knowledge of the structures inside a plant cell are necessary for students to understand the processes involved in photosynthesis. The teacher may choose an on-line version for explaining the structure to students. One such example is found at https://www.youtube.com/watch?v=NTunJq9LtS0
Briefly, inside the cell wall, composed of cellulose (a complex sugar) and an inner semi-permeable plasma membrane, is the cytoplasm of the cell. The cytoplasm contains the cytosol, supporting all the tiny organ systems (or organelles) that carry out the functions for plant growth. These are
• the nucleus, containing the genetic material (DNA) and the nucleolus. The nucleolus contains 2 types of nucleic acids – deoxyribonucleic acid (DNA ) and ribonucleic acid (RNA). As messenger RNA (mRNA), it transmits the genetic message to the ribosomal system outside the nucleus.
• ribosomes are bound to an extensive channel system called the rough endoplasmic reticulum. (rER). Here, with the help of transfer RNA, ribosomes decode the genetic information, and transcribe it into complex molecules, such as proteins and hormones.
• the smooth endoplasmic reticulum (sER) is associated with the production of lipids
• amyloplasts synthesise and store starch in plant cells.
• Golgi apparatus packages the proteins and lipids into membrane-bound vesicles for them to be transported outside the cell. Named after its discoverer, Camillo Golgi, the Golgi body appears as a series of stacked membranes.
• chloroplasts – these are the organelles that capture the Sun’s energy in glucose
• mitochondria – these organelles, using oxygen, produce the energy from glucose (in the process called cellular respiration). Plant cells need the energy to carry out their function
Activity: Have students model a plant cell and include all organelles.
Resource:
Activity
Show the Effect of Sunlight on Leaves:
(Courtesy, Xavier Rodrigues, Shenton College)
MATERIALS
• Gravel or small pebbles
• Activated carbon
• Soil
• Leafy plants
• 2 bottles or glass containers, with lids.
• Tweezers
• Sphagnum moss
• spray bottle (optional)
METHOD
1. Cover the base of the container with the gravel (or small pebbles)
2. Add a fine layer of activated carbon on top of the gravel.
3. Compact a thick layer of soil onto the container.
4. Using tweezers, carefully plant the plant into the soil. You should be pushing the roots into the soil.
5. If you have Sphagnum moss, compact a thin layer on top of the soil. Ensure you are not damaging or covering your plants when you do so.
6. Spray some water into the container. Do not spray too much as you will flood the ecosystem!
7. Tighten the lid.
8. Place one container in sunlight (this can be indoors), and the second container away from sunlight (in a dark place).
9. After several weeks, examine the containers.
RESULTS
• Those placed in sunlight will have leaves that are green, and moisture on the inside of the glass; an indication that transpiration has occurred.
• Those placed in the dark will have yellowing leaves, and moisture on the inside of the glass, but possibly less. This is because photosynthesis uses only a small proportion of water drawn up from the soil by the plant; most of it passes out through the leaves as transpiration.
CONCLUSION
The yellowing of leaves indicates a lack of the Sun’s energy, as photons/rays, entering the chloroplasts inside the leaf’s cells.
RECORD
Write up your experiment, by recording any differences between the 2 plants, provide an explanation and attach any photographic evidence.
Terrariums. Photo Xavier Rodrigues
Activity
Represent Photosynthesis as an Annotated Equation:
How it all Works
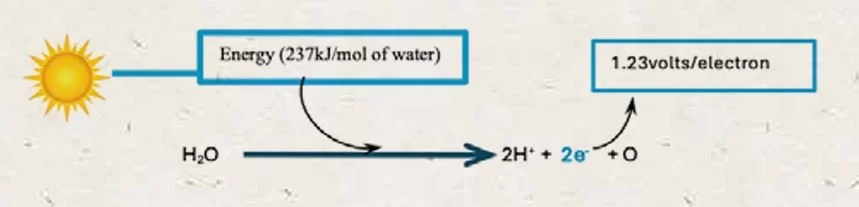
1. Splitting Water
Light energy (photon) splits the water molecule, energising the electrons when hydrogen is separated from oxygen.
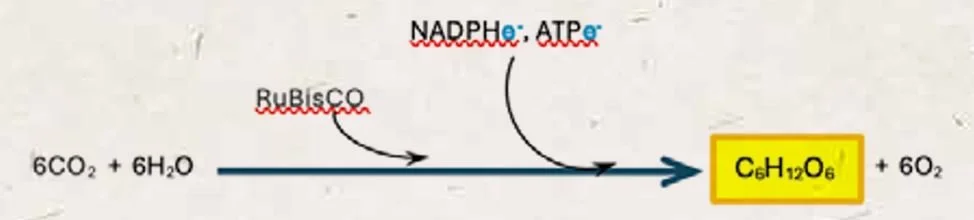
2. Fixing Carbon Dioxide Into a Sugar
In the stroma of the chloroplast is an important enzyme with a long name, ribulose-1,5-bisphosphate carboxylase/oxygenase, or RuBisCO (see GLOSSARY). Together with the energised molecules ATP and NADPH, that supply the energy, RuBisCO catalyses carbon dioxide, through a series of steps, into glucose.
Cellular Respiration
Converting Energy From Sugar
The equation is reversible. When animals (and plants) require energy for processes such as reproduction, movement, metabolism, etc. , the potential energy stored in glucose becomes kinetic energy when glucose is broken down into its constituents, carbon dioxide and water, a process called cellular respiration.
• During cellular respiration, glucose (a 6-carbon molecule), with the help of an enzyme, is first broken down to pyruvic acid, a 3-carbon molecule.
• Transported into the cell’s mitochondria, pyruvic acid is oxidised to form pyruvate, during which 2 molecules of ATP are energised. Pyruvate is further broken down in a cycle of chemical reactions, the Krebs Cycle, named after its discoverer, Hans Krebs.
• Here, the main energy carrier is NADH with its energy-rich electrons. Inside the mitochondria is a series of molecules called cytochromes. NADH passes its electrons down the cytochrome chain, in the process, energising about 28 mol of ATP (from 1 mol glucose). The energy in ATP (carried in its terminal phosphate bond) is then able to power up all cellular processes that require energy.
Note that a small quantity of water is produced, called metabolic water. It is the only source of water in some animals, such as a desert rodent that is able to survive with no source of external water. The animal’s requirement is met from the food it eats (2).
Activity
Be a Leaf and Make Glucose:
Show Powerpoint – Plants and visible light, Part 2 (TOOLBOX)
Students are introduced to a molecule and an atom, and model glucose from its constituent atoms.
MATERIALS
(courtesy Ben Tisdale, Bullcreek PS)
• 3 colours of modelling clay (plasticine from Officeworks) – yellow, red and blue. Quantities are calculated for 30 students, working in pairs. You will need 1 x 500g block of yellow, 2 x 500g blocks of blue, and 3 x 500g blocks of red. This will be sufficient for a class of 30 students, working as 15 pairs.
• Wooden skewers, cut into 5cm lengths, or Natural Matchsticks (without ignition) from Teacher Superstore ($17.95 for 5,000).
Teacher Note. This activity has been performed with marshmallows of similar colours (Yr 6, Mt Pleasant PS).
METHOD
1. Hydrogen (blue), carbon (yellow) and oxygen (red) ‘atoms’ may be modelled by the student pair. Each pair will need 6 x 3g balls of yellow, 12 x 3g balls of blue and 18 x 3g balls of red. Have balls prepared prior to the class.
2. Their weight is not exact, but balls should be approximately the same size.
3. Distribute WORKSHEET – Building glucose (TOOLBOX)
4. Show the PowerPoint What a Leaf Can Do with Sunlight on the class screen, introducing the Sun as the source of energy.
5. Step down through the 12 steps, guiding the class in constructing a glucose molecule.
6. Record in the WORKSHEET with each step.
7. By Step 13, students discover that the leaf has also made oxygen.
8. Display the glucose models in the classroom.
9. Show the Movie – Glucose Molecules – performed by Yr 5-6 Mt Pleasant PS (TOOLBOX)
Glucose Molecules Yr 6, Mt Pleasant PS.